The removal of personal data (such as names or addresses of clinical trial participants) so that people using clinical trial data cannot identify the individuals who took part. Truly anonymised data contain no information that could reasonably be used, by anyone, to identify individuals even by cross-checking the data against other sources of information.
- EUPATI – Accademia del Paziente esperto (AdPEE) Toolbox Glossary: https://toolbox.eupati.eu/glossary/anonymise
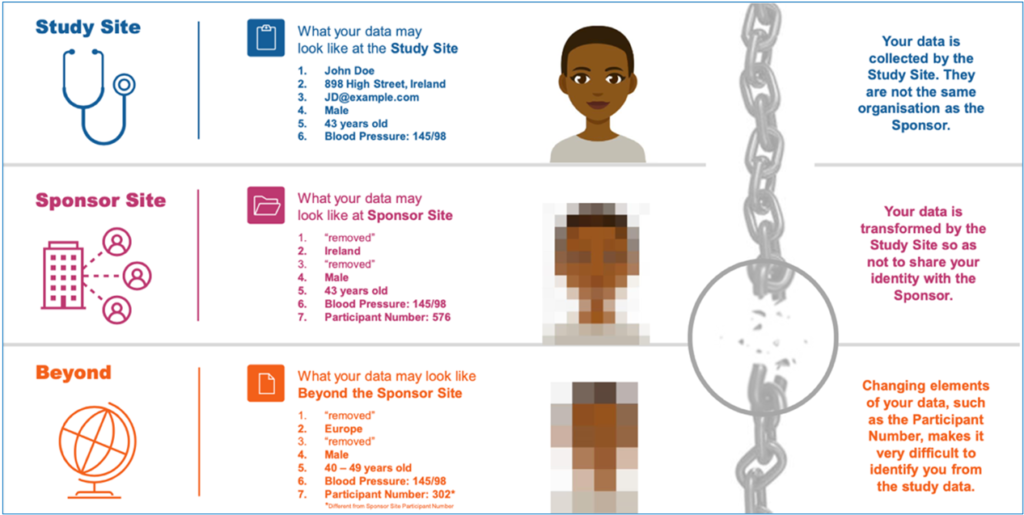
Courtesy of TransCelerate project (https://www.transceleratebiopharmainc.com)
See also
More resources
- Terminology Harmonisation in Data Sharing and Disclosure Terms and Definitions (version 2): https://phuse.s3.eu-central-1.amazonaws.com/Deliverables/Data+Transparency/WP066.pdf
- Multi-Regional Clinical Trials-MRCT Center of Brigham and Women’s Hospital and Harvard Glossary: https://mrctcenter.org/clinical-research-glossary/glossary-words/anonymize
Video
Anonymising patient data for research: How does that work
This video, realized by the National Institute for Health and Care Research (NIHR). University College London Hospitals Biomedical Research Center, shows how the process anonymization works and its implications for clinical research.
Source: National Institute for Health and Care Research (NIHR). University College London Hospitals Biomedical Research Centerhttps://www.ucl.ac.uk/health/working-partnership/nihr-biomedical-research-centres



