An analyzable clinical trial dataset is a set of data that, after collection (raw data), is stored into an organized data management system for further evaluation and processing. Clinical trial raw data typically undergo a process of cleaning, quality assurance, and quality control.
This is to detect inconsistent, incomplete, or inaccurate entries, and to confirm that the data were collected and evaluated according to the protocol and match the source data (analyzable dataset).
This process of data control continues over the course of the trial as data are collected and afterward. Both the raw data and the analysable dataset are individual personal data (IPD).
- Sharing Clinical Trial Data Maximizing benefits, minimizing risk | Chapter 4. The Clinical Trial Life Cycle and When to Share Data. National Academy Press (NAP): https://www.ncbi.nlm.nih.gov/books/NBK286004/
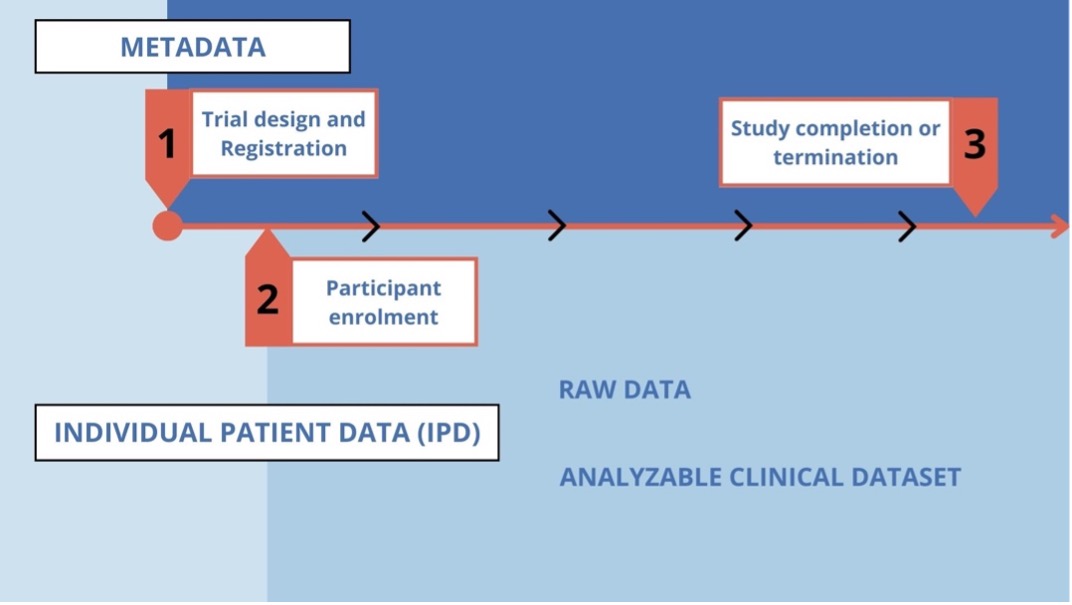
Adapted from: Sharing Clinical Trial Data Maximizing benefits, minimizing risk | Chapter 4. The Clinical Trial Life Cycle and When to Share Data. National Academy Press (NAP). https://www.ncbi.nlm.nih.gov/books/NBK286004/



