Electronic-Consent (e-Consent) is a method of obtaining informed consent using an electronic system instead of a paper consent form. The consent process does not change, the same consent process steps are still applicable.
- The Multi-Regional Clinical Trials Center of Brigham and Women’s Hospital and Harvard (MRCT). Glossar: https://mrctcenter.org/glossaryterm/e-consent-form/
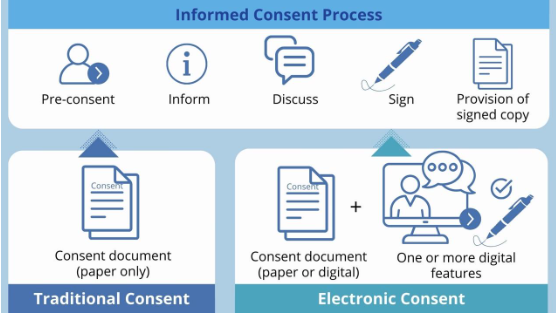
Source: European Forum for Good Clinical Practice (EFGCP) e-Consent Initiative Glossary of e-Consent Terms: https://efgcp.eu/public
More Resources
- NCI Thesaurus. Electronic Consent form: https://ncithesaurus.nci.nih.gov/ncitbrowser/ConceptReport
To go in depth
e-Consent and the Covid-19 Pandemic
An example of how e-Consent has been implemented in a UK Clinical Trials Research Unit providing an overview of the critical issues to solve for an efficient implementation.
- Cragg WJ et al. Approaches and experiences implementing remote, electronic consent at the Leeds Clinical Trials Research Unit. Trials. 2024; 25:310: https://trialsjournal.biomedcentral.com/counter/pdf/10.1186/s13063-024-08149-y.pdf
The good and the bad of an e-Consent
A link to TransCelerate Initiative that explains e-Consent is, what are the characteristics of an e-Consent form, including advantages and disadvantages
- What is e-Consent? TransCelerate Biopharma Inc.: https://www.transceleratebiopharmainc.com/assets/econsent-solutions/what-is-econsent/
Video
All you need to know about Informed Consent and e-Consent
This video realized by the Center for Information and Study on Clinical Research Participation’s (CISCRP) provides a clear overview on Informed Consent and e-Consent: process of learning and agreeing to be in a clinical trial, who to speak with and ask questions before participation. It also focuses on the choice, after giving consent, to stop participating at any time for any reason, understand the purpose, length, risks, benefits and what will happen during the clinical trial.
Source: Center for Information and Study on Clinical Research Participation’s (CISCRP) https://youtu.be/3gNo8_PVLA0



