The Lay Summary (LS) is a short summary of clinical study results using plain language to inform the public. It usually spans some 5-10 pages and describes a clinical study’s key features, including the study objective, rationale, demographics, efficacy, and safety results. The European Clinical Trial Regulation (CTR) specifies 10 essential elements that must be included in a lay summary.
In the EU, this document is to be provided by the sponsors after the end of each clinical trial in the EU: no later than 12 months from the protocol-defined end of the clinical trial, 6 months for pediatric studies, and up to 30 months for non-therapeutic Phase 1 trials.
Several terms have been used to describe the same document summarizing clinical trial results in a way as to be understandable to the lay public. Various organizations – such as the Multi-Regional Clinical Trial (MRCT) working group, TransCelerate, FDA and EU CTR – use different terms as synonymous:
- Research Results Summary (RRS)
- Layperson Summary (LS)
- Plain Language Summary (PLS)
- Plain language trial summaries (PLTS)
- Schindler TM. Lay Summary of Clinical Trials results. Regulatory Writing: An Overview, 2nd edition, Regulatory Affairs Professional Society (RAPS), December 2020, Lisa DeTora (editor), 265-277: https://www.researchgate.net/publication/347445255_Lay_Summaries_of_Clinical_Study_Results/link
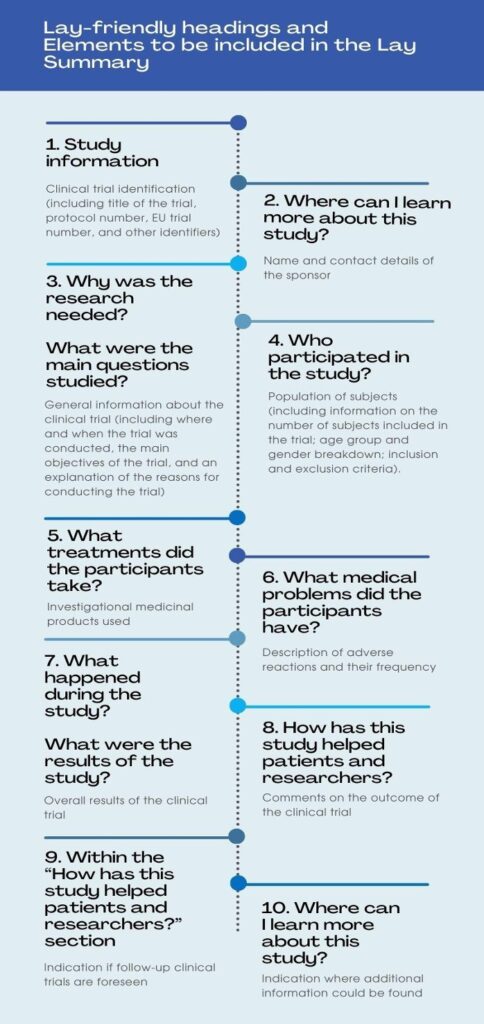
Source: Barnes A, Patrick S. Lay Summaries of Clinical Study Results: An Overview. Pharmaceutical Medicine 2019; 33: 261-268: https://link.springer.com/article/10.1007/s40290-019-00285-0#Tab1
To go in depth
Good practice in lay summaries
The Good Lay Summary Practice (GSLP) guidance provides recommendations on how to prepare, write, translate, and disseminate summaries of clinical trial results in lay language
- Good Lay Summary Practice (GSLP) Guidance, Version 1, 4 October 2021: https://health.ec.europa.eu/system/files/2021-10/glsp_en_0.pdf
Lay Summary of Clinical Trials results: an article
This article defines lay summaries for clinical study results, their regulatory basis, their content, open questions, and new developments. It summarizes current guidance as well as regulatory differences between the EU and the US.
- Schindler TM. Lay Summary of Clinical Trials results. Regulatory Writing: An Overview, 2nd edition, Regulatory Affairs Professional Society (RAPS), December 2020, Lisa DeTora (editor), 265-277: https://www.researchgate.net/publication/347445255_Lay_Summaries_of_Clinical_Study_Results/link
The European Patients Forum (EPF)’s position
This position statement of the European Patients Forum (EPF)’s focuses on the importance of communicating scientific research to patients and public, explaining what kind of information should be provided in a language and visual presentation that makes it easy to understand for lay persons.
- European Patients Forum (EPF)’s position: Clinical trial results – Communication of the lay summary: http://www.eu-patient.eu/globalassets/policy/clinicaltrials/epf-lay-summary-position-final_external.pdf
A useful overview, with some practical examples
This article provides a comprehensive review of what are the requirements of a lay summary, by presenting concrete examples of the elements required by European legislation.
- Barnes A, Patrick S. Lay Summaries of Clinical Study Results: An Overview. Pharmaceutical Medicine 2019; 33: 261-268: https://link.springer.com/article/10.1007/s40290-019-00285-0#Tab1



