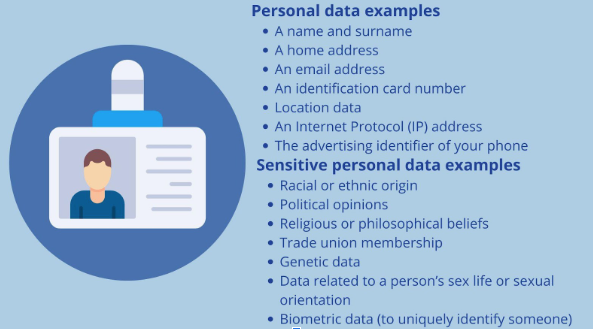
It refers to any information that relates to an identified or identifiable individual, either directly or indirectly. In a clinical setting, personal data includes demographic details (such as name, age, and contact information), medical history, treatment records, and any other information that could be used to identify an individual. The handling of personal data is governed by regulations, such as the General Data Protection Regulation (GDPR), which emphasize the need for privacy, consent, and security in its collection and processing. Sensitive personal data, also known as special category data, is a specific set of “special categories” that must be treated with extra security.
- European commission directorate-general for health and food safety. Question and Answers on the interplay between the Clinical Trials Regulation and the General Data Protection Regulation: https://health.ec.europa.eu
Video
GDPR and Clinical Trials: The Role of a Data Protection Representative
The following video, realized by GCP-Mindset, All About Clinical Research explores discover the crucial role of a Data Protection Representative in ensuring GDPR compliance in clinical trials.
Source: GCP-Mindset, All About Clinical Research. Data Protection Representative



