Clinical trial data originates from patients and healthy volunteers who participate in studies. Raw data (sometimes called source data) are observations about individual participants used by the investigators in a clinical trial. At the source, these data may be in the form of measurements of participant characteristics such as weight, blood pressure, or heart rate and may be associated with the baseline (or initial) visit or subsequent follow-up visits.
Raw data may also include a baseline description of the participant’s medical history, physical exam information, clinical laboratory results (e.g., serum lipid values, hemoglobin levels), whole exome or genome sequences, imaging results (e.g., X-ray, magnetic resonance imaging [MRI]), procedure results (e.g., electroencephalogram [EKG], endoscopy), or self-reported data (e.g., symptoms, quality of life).
Raw data is collected between the time of first participant enrollment and study completion. During the trial, the raw data is abstracted, coded, and transcribed. After participant activities have ended, the data are cleaned into an analyzable data set. Both the raw data and the analyzable data set are individual participant data.
- Sharing Clinical Trial Data Maximizing benefits, minimizing risk Chapter 4. The Clinical Trial Life Cycle and When to Share Data. National Academy Press (NAP): https://www.ncbi.nlm.nih.gov/books/NBK286004
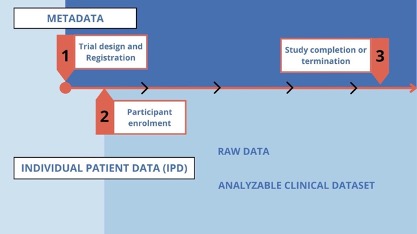
Adapted from: Sharing Clinical Trial Data Maximizing benefits, minimizing risk Chapter 4. The Clinical Trial Life Cycle and When to Share Data. National Academy Press (NAP)
More resources
- CDISC Clinical Research Glossary: https://www.ecfs.eu/sites/default/files/general-content-files
- Sharing Clinical Trial Data Maximizing benefits, minimizing risk Chapter 4. The Clinical Trial Life Cycle and When to Share Data. National Academy Press (NAP): https://www.ncbi.nlm.nih.gov/books/NBK286004.



