At the 9th Conference of the European Association of Health Law, held at the University of Warsaw from September 18-20, Wenkai Li, a researcher from Vrije Universiteit Brussel, delivered an insightful presentation titled “Rethinking individual autonomy in the secondary use of health data for research: A legal and ethical analysis on informed consent”.
Li’s presentation was informed by the experiences and key findings from the FACILITATE project, focusing on the normative complexities of reusing clinical trial data for research purposes. Central to his talk were discussions on rethinking the normative frameworks governing health data and the need for reconciliation between legal requirements and ethical considerations in this field.

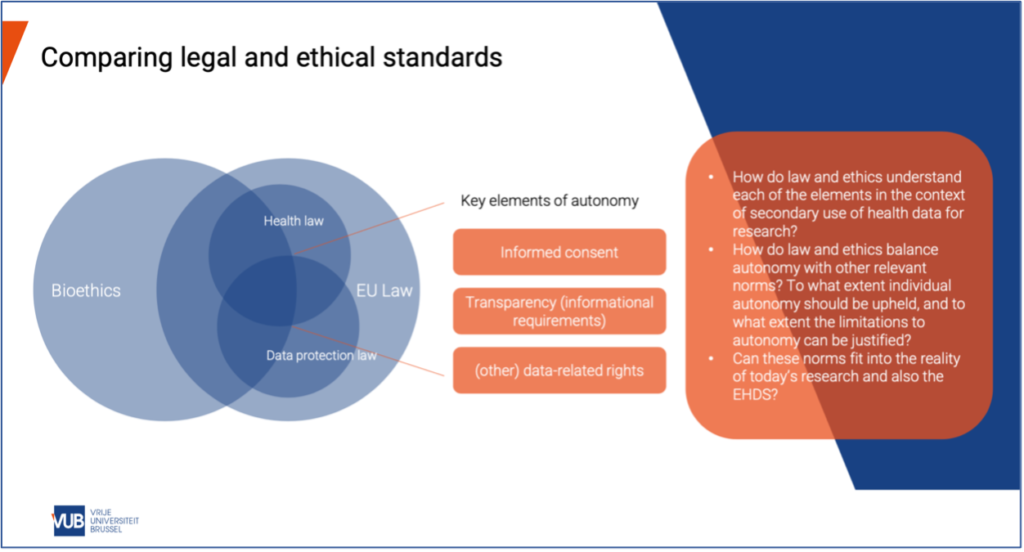
Currently, how to reconcile law and ethics in the secondary use of clinical trial data is a central debate. Li emphasized the particular relevance of bioethics, health regulation, and data protection law in this context.
Another point of discussion was the overlapping but nuanced ethical and legal standards, particularly with regard to understanding informed consent.
Finally, Li considered the delicate balance between the interests of study participants, clinical trial sponsors, health care providers, and researchers (data users).
Below, the main takeaways of Li presentation.
- The reality of data-intensive health research necessitates a rethink of the role of autonomy and related concepts, in particular, informed consent, as part of a new normative framework where a number of interests, values and social goals need to be considered and balanced.
- While the international bio-(medical) ethics guidelines still take a consent-centric approach, other ethical views do not perceive consent as indispensable in the context of secondary use of health data for research.
- While the GDPR generally shows an open attitude for research, legal uncertainty and impediments still persist.
- A moral duty to make data available for health research could provide arguments for the non- consent/opt-out approach taken by the EHDS. However, the legality and ethicality of this approach remain to be examined in terms of the alternative safeguards, accountability and oversight mechanisms, and the overall trust of the public.